YEYETAC™ AeroLock Chest Seals test in Extreme Conditions
- YEYETAC™

- Oct 20, 2025
- 3 min read

Test Objective & Equipment
Our independent testing evaluated how leading chest seal products perform when exposed to Arctic like conditions (-40°C) for extended periods (17 hours), simulating worst-case scenarios faced by military personnel in extreme cold weather operations.
Testing Equipment:
High and Low Temperature Testing Machine (TEMP&HUM calibrated environmental chamber)
Specialized gunshot wound simulation platform with:
Standardized wound aperture matching typical GSW dimensions
Integrated injection device for air and fluid simulation
Pressure measurement systems for quantitative evaluation
Test Methodology:
The testing approach simulated field application conditions using:
Specialized wound simulation platforms replicating gunshot wounds
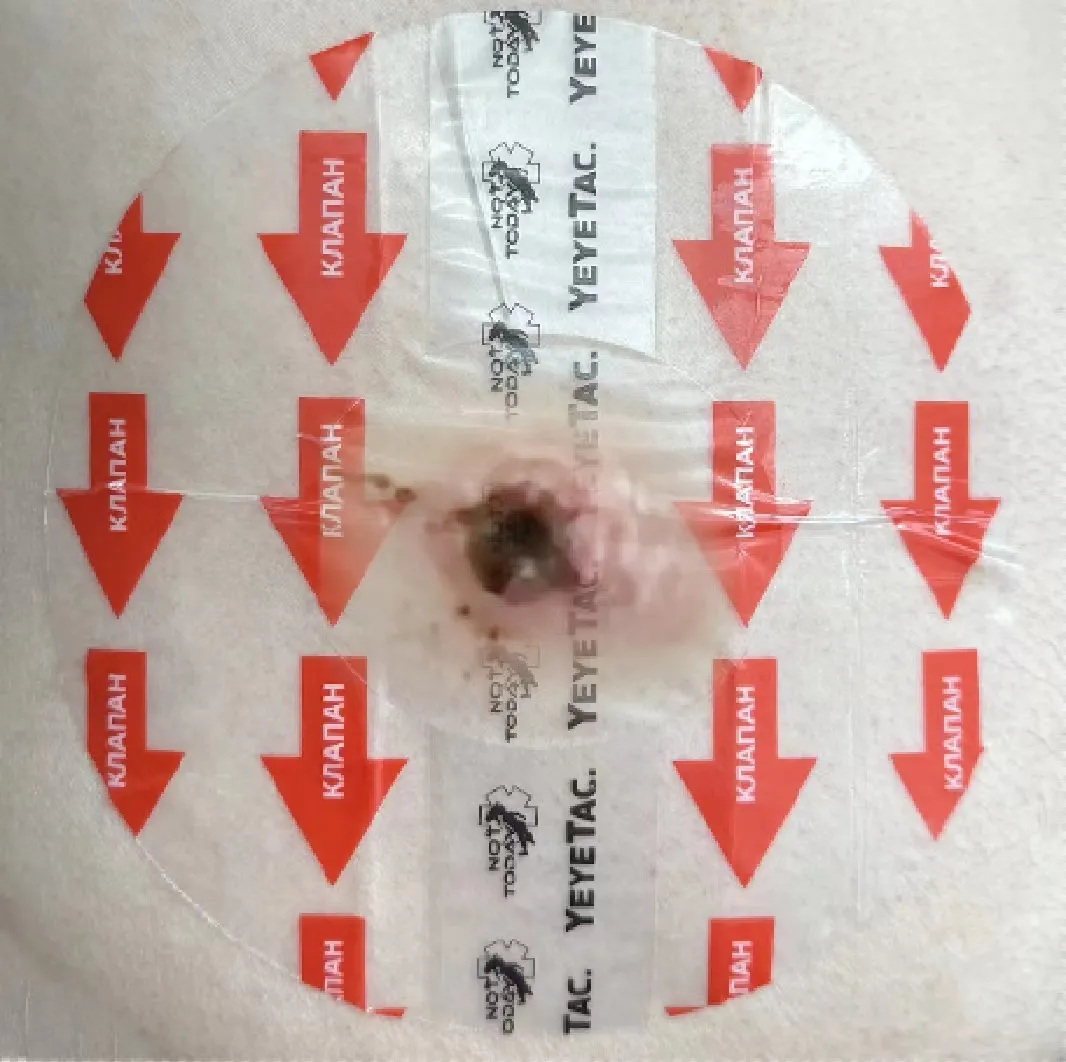
Coffee powder placement on vent channels to provide visual confirmation of air movement
Standardized fluid injection to mimic blood evacuation requirements
Controlled cold exposure at -40°C for a full 17-hour period
Standardized application technique matching tactical field protocols
Comprehensive Test Report Validates Critical Combat Medical Equipment
In tactical combat casualty care, selecting the right equipment can mean the difference between life and death. When treating penetrating chest wounds, understanding how chest seals perform under extreme battlefield conditions becomes crucial for military procurement decisions and operational readiness.
Performance Analysis: The Cold Hard Facts
Our independent testing lab evaluated leading chest seal products at extreme temperatures of -40°C for 17 hours and conditions that mimic some of the harshest operational environments military personnel might face. The findings provide valuable insights for procurement officers tasked with equipping combat medical kits.
Key Performance Metrics at -40°C
Why These Findings Matter for Military Operations
When treating thoracic trauma in combat scenarios, dependable chest seals must maintain functionality regardless of environmental conditions. The data reveals significant performance gaps between premium tactical medical products and generic alternatives:
Adhesion Reliability: Only premium products (AeroLock, Hyfin, SAM) maintained adhesion at extreme temperatures and critical for maintaining an airtight seal during casualty evacuation.
Ventilation Effectiveness: The properly engineered vent channels in military grade seals allowed for effective air and blood release, preventing tension pneumothorax even in extreme cold.
Deployment Considerations: AeroLock's combination of minimal size/weight (16.7g) with reliable performance offers advantages for weight-conscious loadouts.
Accessory Value: Included cleaning gauze in premium products provides additional tactical utility, with Hyfin offering the most comprehensive wound preparation materials.
Implications for Military Procurement
This rigorous testing methodology offers procurement officers objective data for evaluating cost-versus-performance tradeoffs in bulk tactical medical supply purchasing. The dramatic failure of non-brand alternatives demonstrates why specialized battlefield medical equipment commands premium pricing and delivers superior value when lives are on the line.
For European NATO forces operating in Arctic regions or high-altitude environments, these findings provide essential insights for maintaining medical capability under all operational conditions.
Beyond the Numbers: Testing Methodology
The testing protocol utilized a specialized simulation platform replicating gunshot wounds, with injection mechanisms to validate both air and blood release functionality. This approach mirrors real-world battlefield injuries that military medical personnel encounter.
The comprehensive testing process included:
Environmental stress testing in calibrated temperature chambers
Simulated wound application using standardized pressure protocols
Measurement of adhesion strength after prolonged cold exposure
Air release validation using visible indicators (coffee powder movement)
Blood simulation testing with standardized fluid parameters
Complete Testing Documentation Available
This report represents just one component of our comprehensive testing portfolio for tactical medical equipment. Additional testing documentation is available for:
Combat tourniquet reliability assessment
REACH testing of first aid kits
thermal insulation performance testing of emergency blankets
emergency blanket tensile strength testing
Field-deployable wound management systems
Extended environmental performance data
For access to the complete technical documentation or to discuss integration with existing military medical systems, contact our procurement specialist team at support@tacticalmedicalkit.com.
FAQs
Why is extreme cold testing particularly important for military chest seals?
Combat operations occur in diverse environments, from arctic regions to high altitude mountains. Equipment that fails in extreme cold could compromise casualty survival during prolonged field care and tactical evacuation.
What makes military grade chest seals different from civilian models?
Military grade chest seals undergo more rigorous testing for environmental extremes, feature enhanced adhesives that work on wet/bloody surfaces, and typically include specialized venting systems for managing tension pneumothorax under tactical conditions.
How do these findings impact military medical doctrine?
Evidence based equipment selection ensures combat medics and tactical operators can rely on their gear under all battlefield conditions, supporting the golden hour concept for treating preventable combat deaths.
Are chest seals with gauze preferable for battlefield applications?
Integrated gauze pads provide immediate wound preparation capability, reducing treatment time and simplifying inventory for army combat medics and special operations medical personnel.
What are the logistics considerations for bulk chest seal procurement?
Beyond performance metrics, military procurement officers should consider packaging durability, shelf life certifications, and compatibility with standardized IFAK (Individual First Aid Kit) dimensions.






Comments